The production of agar, bacteriological agar and agarose are considered in this section.
Agar manufacturing processes have developed since the early freezing method was used to concentrate the extracts of agarophyte seaweeds. Whichever process is used, the following criteria should be taken into consideration. Firstly, it is necessary to obtain an extract from agarophyte seaweeds that contains the largest possible amount of the existing agar in the agarophytes. Secondly, the agar obtained should have the best possible characteristics to satisfy the standards expected for this product, especially as far as the gel strength is concerned. To achieve this it is necessary to consider the following basic points for the manufacturing process.
1. The seaweed treatment prior to extraction.
2. The control of molecular weight distribution during the extraction.
3. The removal of undesired products.
4. The need to work with large volumes of dilute extracts.
5. The economics of dehydrating the dilute extracts.
1. SEAWEED TREATMENT PRIOR TO EXTRACTION
The seaweed treatments prior to extraction are very important as they will condition to a high degree the characteristics of the agar obtained. For example Gracilaria agar was once called an agaroid because at that time Gracilaria was not preteated properly resulting in a product softer than that obtained from Gelidium. Now Gracilaria is given a strong alkaline treatment before extraction. This causes hydrolysis of sulfate groups and transforms important quantities of L-galactose 6-sulfate into 3,6-anhydro-L-galactose, thereby significantly increasing the gel strength of the agar obtained. Tagawa and Kojima (1972) say the industry uses 0.25-0.5M sodium hydroxide solution at 80-90°C for 3-5 hours. Okazaki (1971) gives more detail, showing how the treatment varies depending on the country of origin of the Gracilaria (Okazaki is a useful reference for details of all methods used in the Japanese agar industry). Yang (1982) gives references to the methods used in Taiwan Province. The treatment, also called sulfate alkaline hydrolysis, must be adapted to the class of seaweed used, to obtain as much desulfation as possible while still avoiding the yield losses that this process can cause. These losses can be very important if agar is dissolved in the alkaline solution. The way these treatments are applied is variable and constitutes a part of the manufacturing process that has to be constantly adapted, according to the changing seaweeds, as it becomes a double-edged tool that can substantially reduce the yield if it is wrongly applied.
2. CONTROL OF MOLECULAR WEIGHT DURING EXTRACTION
Agar, as it occurs in seaweed, when extracted is insoluble in cold water and also practically insoluble in hot water. It is therefore necessary to extract it using suitable pH and redox conditions so that some hydrolysis occurs, thereby increasing its solubility. During this fractionation or cracking, it is necessary to avoid the subsequent reduction, by hydrolysis, of the molecular weight of the fragments which have dissolved. As all manufacturing methods are based on agar being soluble in hot water but insoluble in cold, excessive molecular weight reduction of the agar in solution would cause reduction of yields during the process, whenever molecular weights are reached for which cold solutions are possible. On the other hand it is important to avoid molecular units, in the agarophyte residues, that are not soluble either for lack of the necessary solution time or because of an excessive molecular weight that curtails solution under the conditions of extraction.
Figure 10 attempts to clarify a complex process in a simplified way since what we are putting into solution is not only agarose, with a quite uniform chemical structure, but also a mixture of agaropectins carrying electronegative charges, with a minimum solubility temperature that is above the one for agarose. We can see in the figure that all those molecules with molecular weights below PM1 will be easily extracted from the seaweed but will be lost due to their cold water solubility. In contrast, those molecules that remain in the seaweed with molecular weight above PM2 will not be extracted and will remain with the cellulose residues after extraction. The agar manufacturer has to establish working methods that enable the preparation of a molecular weight distribution curve that avoids both losses as much as possible. An ideal result would be that shown by the middle graph of the three shown in Figure 10. It is very difficult to modify the PM1 value but it is possible to increase the PM2 by raising the water temperature in the extraction; this is done by working under pressure whenever the seaweeds permit it. Naturally the differing stabilities of agars to hydrolysis poses limits to such temperature increases.
The industrial objective aims toward narrowing the type of Gaussian curve shown in Figure 10. This reduces losses and increases the molecular weight to the corresponding maximum in the chart which is accompanied by an increase in the agar gel strength. Such considerations will be correct whenever a constant agarose-agaropectin ratio is maintained.
3. REMOVAL OF UNDESIRED PRODUCTS
During the extraction process, a myriad of undesired products will be obtained as well as agar. Such products are soluble salts, seaweed pigments, cellulose, hemicellulose and many extracts coming from impurities and foreign materials contained in the weed, since commercial seaweeds differ greatly from those with which scientists work. Therefore in order to obtain the purest possible extracts in industry, seaweeds are selected and washed carefully and subjected to previous corrective treatments in which generally an alkaline solution eliminates a large quantity of foreign substances, particularly red pigments (phycoestrine), changing the weed to a green colour. This alkaline treatment is with sodium carbonate; it is milder than the alkaline treatment with sodium hydroxide which is used to improve the gel strength of Gracilaria agar.
A careful filtration will purify the extract but this is quite a difficult operation which requires a high temperature (85-100°C) because of the extract’s viscosity and high gelling power. Also cellulose and seaweed “floridean starch” residues, and even clay particles, make the filtration very difficult. Pressure filters are commonly used. Filter presses are the most useful ones, although modern factories use filters specially designed for this purpose.
Differences in the raw material greatly influence the operating methods and this makes further generalizations impossible.
Figure 9 Agarose gelification
Figure 10 Distribution of molecular weights in agar extracts
4. LARGE VOLUMES OF DILUTE EXTRACTS
Due to the high seaweed cost, high yields of agar are essential. However the extract concentrations range from 0,8% to 1.5% as a maximum; it is difficult to work with a more concentrated extract, for filtration as well as in the rest of the process. So the more agar that is extracted, the more water must be added to keep the concentration in the above range. This means that it is necessary to work with large volumes of extracts.
5. THE ECONOMICS OF DEHYDRATING THE DILUTE EXTRACTS
An important aspect to consider is the economics for dehydrating the large volumes of dilute extracts discussed in (4), This is a characteristic problem for this industry and its solution lies in methods based on the insolubility of agar when the extracts are cooled. Sometimes, because of lack of experience with the industry, projects are encountered in which evaporation or precipitation are recommended as the means of removing the large quantities of water from the extracts. We would first like to show why these methods are not feasible and afterwards discuss the methods actually used by the industry.
A. EVAPORATION
Starting from a 1% extract, 99 litres of water have to be eliminated for each kilogram of agar and since the latent heat enthalpy for water at 100°C is 539 kcal/kg, we need 53 361 kcal/kg (539 x 99 = 53 361). In our calculations we shall compare the heat requirements at a theoretical yield (impossible to obtain and far from the obtainable one) and consider only the heat for change of state; any heat requirement derived from specific heat will not be considered because of its small relative importance.
Working in an evaporator with liquids above a 2% concentration is impossible, problems are also posed by the gelling temperature of the extract and its large non-newtonian viscosity at temperatures close to gelling. All this prevents the thermal savings that could be gained by the use of multiple-effect vacuum evaporators.
B. PRECIPITATION
A working method similar to that used for carrageenan, using alcohol precipitation, could be considered. An economical process using this technique has not been achieved so far but the process is feasible chemically. Agar precipitation in alcohol media is more difficult than for carrageenan because the precipitate is more flocculant (has low cohesion) and is difficult to recover quantitatively. A high heat consumption is required because we have to add the heat needed to evaporate the alcohol, to the 53 361 kcal needed to evaporate the water in the mixture. In addition, the alcohol used for precipitation has to be recovered by distillation for reuse.
If we make our calculations using isopropanol, which is used for producing carrageenan for economic reasons, and consider that we start with an azeotropic mixture, previously recovered, of 87% by weight we are forced to work at least 3 litres of azeotropic isopropanol for each litre of extract to be precipitated. Assuming that such a mixture has a density of 0.8234 kg/L, then for each kilo of agar it would be necessary to evaporate:
99 kg of water extract;
22.5 kg of azeotropic isopropanol.
This second item is composed of 213 kg of isopropanol and 41.5 kg of water. The latent heat enthalpy for isopropanol is 175.8 kcal/kg. Therefore the theoretical heat energy consumption would be:
water: 539 x (99 + 41.5) = 75 729 kcal;
isopropanol: 175.8 x 213 = 37 445 kcal.
This means an energy consumption of 113 174 kcal/kg of agar which is double the heat energy need to evaporate the water contained in the extract, and all of this without taking into consideration the need to concentrate the used alcohol back to the azeotrope plus the recovery of isopropanol vapors that entail a considerable amount of energy. From this it can be seen that the precipitation/dehydration process analogous to that used for carrageenan has a high energy consumption when applied to agar.
For agar, concentration methods are based on its insolubility when cooled and are used in all factories according to two basic principles: freezing or syneresis under pressure.
C. FREEZING
This consists of freezing and thawing the extract, previously gelled, and profiting from the insolubility of agar in the cold to eliminate the greatest part of the water contained in the extract. Freezing should be slow, to allow both the growth of ice crystals and the separation of agar in the highest possible concentration; this is usually followed by draining with a water-extracting centrifuge. Only slow freezing permits large ice crystals to be formed, surrounded by fine sheets of agar. Efforts to speed up freezing produce spongy masses, with high water content and less agar concentration, that dialyse poorly and produce an impure agar, because the impurities which are soluble in cold water do not move so well from the gel to the water.
As far as the economics for this process are concerned, we should consider that if we start with a 1% agar extract, we have to eliminate 99 litres of water per kg of agar; after melting and draining, this agar at best reaches a dry extract content of 15% (1 kg in 6.66 L) but is normally 11-12%. Presuming a 15% agar in the product, the cycle of freezing-defrosting eliminates (99 L – 6.66 L) 92.34 litres water per kg of agar. Furthermore the energy consumption for freezing 1 L or 1 kg of water is 79.67 kcal. To freeze the 99 litres of water contained in the 1% extract would require:
99 x 79.67 = 7 887 kcal
To remove the water remaining in the melted and drained agar requires a heat consumption of:
6.66 x 5 390 = 3 590 kcal
We can see the difference between the sum of these figures (11 477) and those for:
evaporation method = 53 361 kcal,
precipitation method = 113 174 kcal.
Naturally there is a cost difference between obtaining a difference of a kilocalorie by heating or cooling but the figures leave us in no doubt (even though we have ignored the energy consumption derived from the specific heat of the water that is eliminated) there are enormous energy differences between the working methods considered.
D. SYNERESIS
Syneresis is usually described as the process in which a gel contracts on standing and exudes a liquid. Here the term syneresis is used to describe the process where pressure is used to exude liquid from the gel. The water that soaks the colloidal net of the gel is eliminated by applying, by suitable means, a force that will favour such loss. Energy consumption is very low when working in these conditions but not everybody can benefit from it because the industrial technology is not simple. Pressure has to be applied very carefully to avoid gel losses by extruding the gel through the containing system. The advanced factories that use this process have been obliged to develop a very specific technology, not only producing extracts in the appropriate conditions for good syneresis but also equipment design that will allow the efficient treatment of large quantities of extracts.
Initially long syneresis periods were required, with cycles longer than 24 hours, that would start with a gradual and slow increase in pressure by placing, successively and at a prefixed rate, stone blocks on top of the gel containers; the agar gel was wrapped in canvas cloths and placed in a series of steel boxes fitted between the fixed and movable heads of a vertical hydraulic press. This treatment was followed by hydraulic pressing, once the product was consistent enough to withstand extrusion. Usually a modified platen press is used which is similar to a box press but the cloth bags are not enclosed on the sides during pressing and the press is usually built in horizontal form. Nowadays some agar manufacturers have designed their own modern equipment which permits this syneresis to be carried out automatically in relatively short periods of time and operating with large volumes.
Starting with a 1% agar extract, syneresis increases concentration to a maximum of 25% (1 kg agar per 3 L water). If we consider an average of 20% for the dried extract from industrial runs, the heat energy necessary to remove the rest of the water will be:
4 x 539 = 2 156 kcal/kg
which is much less than the heat energy needed to dry the agar obtained by freezing where moisture was calculated in ideal conditions, that are difficult to obtain in reality.
Compared to freezing, syneresis results in large electrical energy savings as the electrical energy needed to maintain a pressure on a quite incompressible product is much less than that necessary to freeze 99 litres of water for each kg of agar produced. The cost of electrical energy makes many freezing factories increase extract concentration but this is possible only up to 1.5% before producing a harmful effect from yield losses. Syneresis, when properly applied, will also produce a purer agar, eliminating a larger quantity of soluble matter.
6. GENERAL
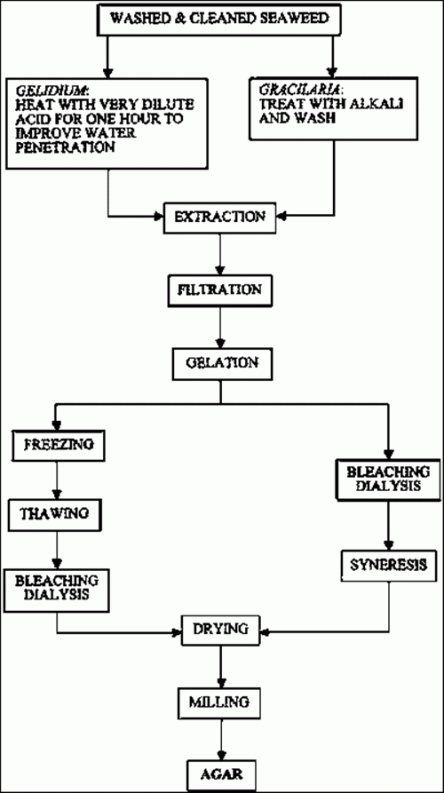
Figure 11 is a flow chart showing the steps used in both of the dehydration processes used to produce agar. Treatment and reagents used in each case will be very variable depending on the species of seaweed used, its origin and even the time of the year when it was harvested. All these factors can cause drastic modifications to the treatment.
 |
| Figure 11, Agar production diagram |
Nevertheless, we should consider some general rules. Seaweeds such as Gelidium, Pterocladia and Gelidiela can be Created by different diffusions, the most usual ones being sodium carbonate solutions, at about 80-95°C. Other reagents such as calcium or aluminium hydroxides or salts can also be used for several purposes. Other treatments with sodium hydroxide solutions of very variable concentration can be used, but the concentration will vary depending on what purposes they are for. As far as Gracilaria is concerned, 0.1 M sodium hydroxide solutions are commonly used; higher concentrations can also be used. The reagents named “Reagents I” in Figure 11 are basically the ones mentioned above. The ones shown as “Reagents II” are Chose used to adjust the extracting conditions and, in general, are organic or inorganic acids or salts with which pH and other extraction parameters are fixed.
The variables in the manufacturing process make it hard for a factory to change the seaweeds it uses as raw materials. Agar manufacturing history is full of fiascos caused by industries trying to change their seaweeds without having adequate technology to adapt to the change.
Water consumption in an agar factory varies widely depending on the seaweed used but it is always very high. Normally factories working Gracilaria seaweeds have a higher water consumption than others. Consumption also increases when an agar of better quality is required, although, in general, it can be reduced by a suitable design of the factory; however this can lead to an increase in investment and therefore to a more difficult project profitability. Factories using the freezing process have very high water consumption as cooling water is needed for the freezing equipment.
Using recycled water, after appropriate treatment, would reduce its consumption but, in general, would increase the plant operating cost. If poor quality water is going to be used, a prior treatment will be required but it is very important to know its cost before the location is decided since a mistake in this point could make the operation of the factory economically impossible.
The above-mentioned problems about water, and those originating from changing to seaweeds of a different origin, are the ones which have led many factories to bankruptcy.
A manufacturer of good quality agar must be ready to monitor his process and so be able to spot readily any variations that seaweeds cause in the yield or in the quality of the final product. For this purpose a well equipped control laboratory is required together with a pilot plant that will enable any modifications needed in the process to be studied prior to the industrial treatment of each batch of raw material. An adequate pilot plant can process from 1-10 kg of seaweeds, depending on the size and importance of the factory. In general small factories with elementary technology do not achieve international quality standards and their products have to be sold at lower prices in local markets. Bacteriological contamination particularly is usually too high and sometimes dangerous in such plants, closing them to many markets.
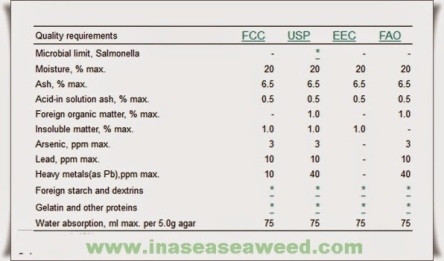
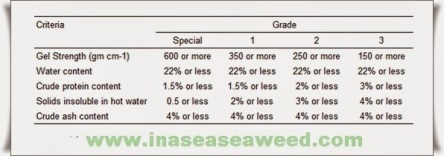
A food grade agar should have a moisture content of less than 18%, ash below 5%, gel strength above 750 g/cm2 (Nikan-Sui method) and a bacterial count below 10 000 bacteria per gram. Escherichia coli and Salmonella must be absent (other pathogenic bacteria may also be specified). Usually the lead content is specified as less than 5 ppm and arsenic less than 3 ppm. These specifications are for agar produced on an industrial scale. In the Orient, large quantities of “natural agar” are sold by very small producers and consumed in the form of threads (“strip”) or bars (“square”) that are usually produced from Gelidium and do not have to meet the above-mentioned specifications. Generally its gel strength is 450 g/cm2 by the Nikan-Sui method.
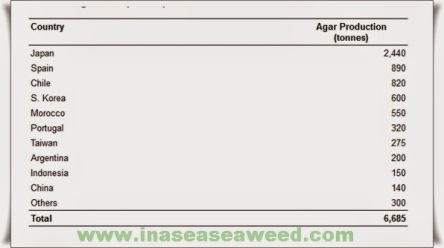
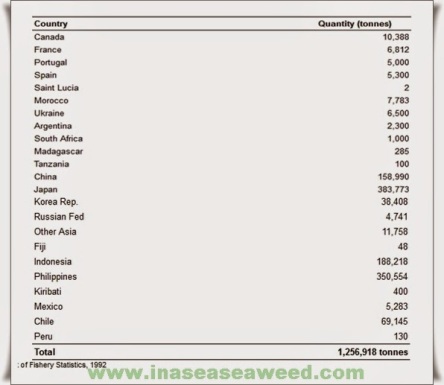
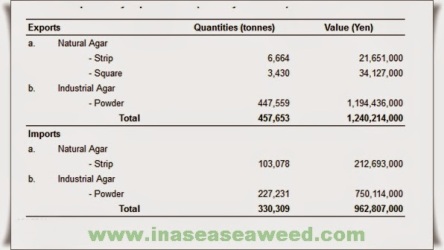
Figure 4b shows as closely as possible what we consider the present situation for the world production of agar. This table has been prepared taking into account the results obtained from an enquiry made among the most important agar manufacturers in countries such as Spain, Chile, Morocco, Portugal, Argentina, Mexico, France, New Zealand, Brazil, etc., and the available Japanese statistics. All these data along with others from Korea, People’s Republic of China, its Taiwan Province and Indonesia have been updated during the XIIth International Seaweed Symposium held in Brazil, August 1986.
BACTERIOLOGICAL AGAR
The use of agar in bacteriology is one of the most important uses and requires strict physical-chemical control as well as the absence of hemolytic substances and what is more important and difficult, the absence of any bacterial inhibitors. Robert Koch started using agar in 1881 to gel culture broths when preparing solid culture media and this was the first introduction of this oriental product to Europe.
Its uses in microbiology are based on the special properties: a gelling temperature of 32-36°C, a melting temperature of 85-86°C, a lack of hydrolysis by bacterial exoenzymes and its ability to be prepared without bacterial inhibitors. The above temperatures refer to culture media gelled with agar and which contain 10-11 g agar per litre of culture media.
Bacteriological agar is prepared from Gelidium and Pterocladia because Gracilaria and Gelidiella give agars with gelling temperatures above 41°C. It is manufactured in a limited number of highly specialized factories and under rigid physical-chemical and bacteriological controls.
There are no specifications for a universal application for bacteriological agar as the different microbiological schools evaluate the parameters in various ways. There are neither international nor national specifications. There are many differences between food grade and bacteriological grade, in physico-chemical and bacteriological controls, but this information is confidential and is shared only by the bacteriological agar and culture media manufacturers.
As agar is used only as a gelling agent in solid media, it is essential to avoid interactions with the rest of the media components such as meat extract, peptones, proteins, amino acids, sugars and other carbohydrates, as well as pigments, indicators, inhibitors, mineral salts, etc., used in their formulation. It has to mix with these components without producing problems such as colour changes, precipitate formation or gel strength losses, even after autoclave sterilization. Therefore actual specifications are different depending on each user and each culture media manufacturer. In general, bacteriological agars are very transparent agars in solution as well as in gel form and they represent the purest qualities in the world market. The rest of the parameters vary as the agars are adapted to the individual requirements of the manufacturer and end user.
In much smaller quantities, and at a much higher price, another type of agar called “Purified Agar” is also available. These are bacteriological agars that could also be used in biochemistry for electrophoresis or immunodiffusion; they can be considered as agarose forerunners, being still used for economic reasons.
Source: FAO